There’s an internet joke that when men are born, they must choose one show to call their own: Breaking Bad, The Wire, or The Sopranos.All three are crime masterpieces, but Breaking Bad stands apart because it somehow made chemistry cool — beakers, formulas, explosions and all. It gave us that immortal scene where Walter White looks his wife dead in the eye and says: “I am the one who knocks. I am the danger.”This year, there are three scientists who are saying the same thing — only this time, they’re here to save people from the danger.And not just people — the planet itself.
So, say hello to the men who turned chemistry from Breaking Bad into Breaking Good. This year’s Nobel Prize in Chemistry is actually pretty easy to get — and no, it doesn’t involve blue crystals or an RV in the desert. The 2025 Nobel went to Richard Robson, Susumu Kitagawa, and Omar Yaghi for inventing something called metal–organic frameworks, or MOFs. If that sounds like chemistry jargon designed to make you feel stupid, relax. MOFs are basically sponges made of metal and carbon, but on a molecular level — invisible, perfect, and outrageously clever.
So, What the Hell Are MOFs?

Think of a MOF as a molecular Airbnb. It’s a structure full of tiny rooms, and gas molecules — like carbon dioxide, oxygen, or even water vapour — can check in and check out whenever they like. Each room is built from a combination of metal atoms (the “metal” part) and organic molecules (the “organic” part), linked together in intricate geometric patterns.They don’t look like sponges, but they behave like one — except their pores are a billion times smaller, precise down to the atom. Imagine a high-tech cage that can trap pollutants, store hydrogen, filter water, and even harvest moisture from desert air.That’s a MOF.
The Guy Who Started It: The Professor with Wooden Balls
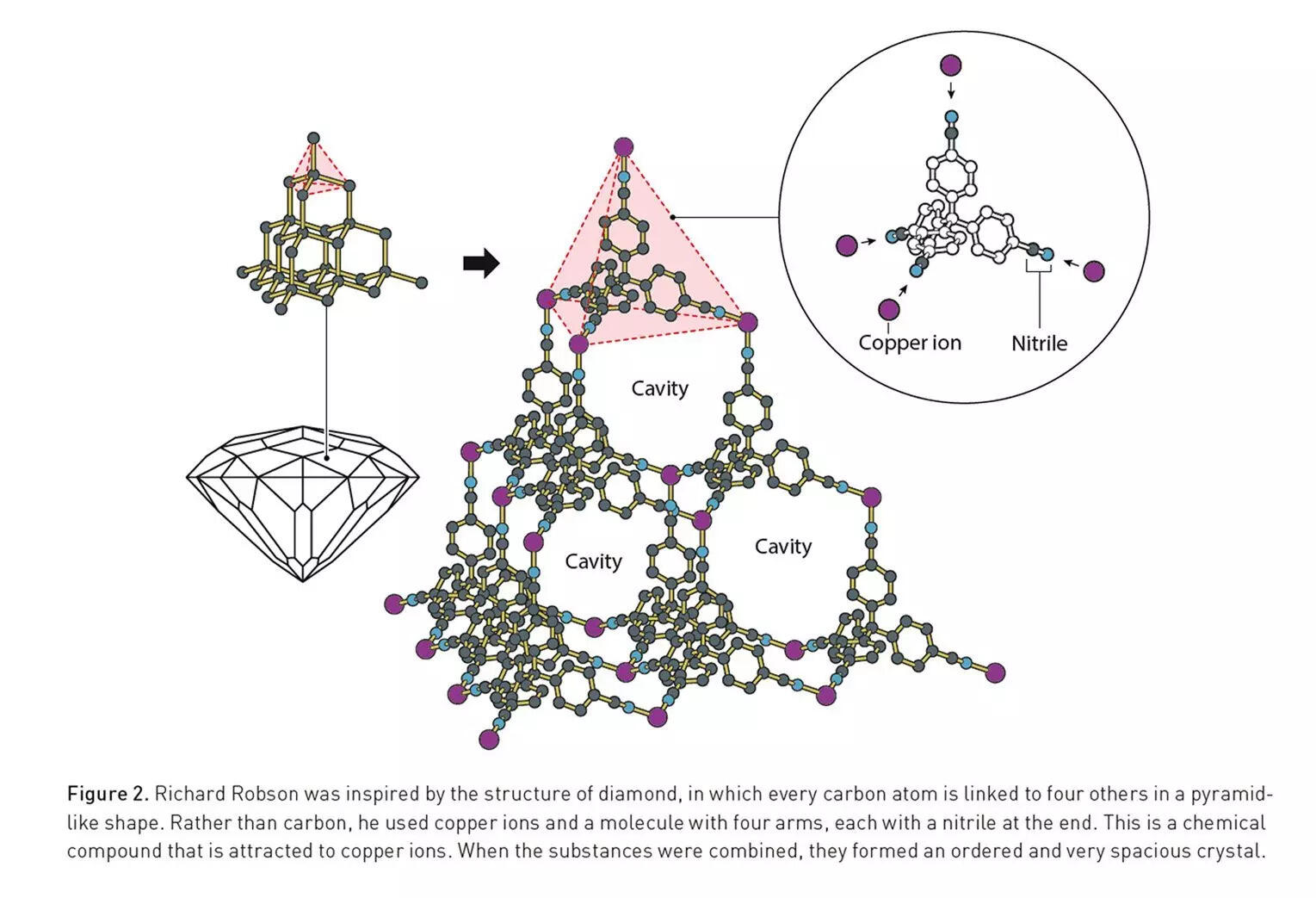
In the 1970s, Richard Robson at the University of Melbourne was teaching chemistry using old-school ball-and-stick models. One day, while staring at those wooden atoms, he realised something: the placement of holes (where the sticks connect) determined what the molecule would become.So he thought — what if we used real atoms that could naturally fit together, like Lego pieces?In 1989, he mixed copper ions with a four-armed organic molecule — and instead of chaos, he got an orderly 3D crystal full of empty space. Like a diamond made of air pockets.Most chemists dismissed it. But Robson had just built the first-ever metal–organic framework. In essence, he gave chemistry its first molecular architecture with a floor plan.
The Philosopher Who Believed in “Useless Things”
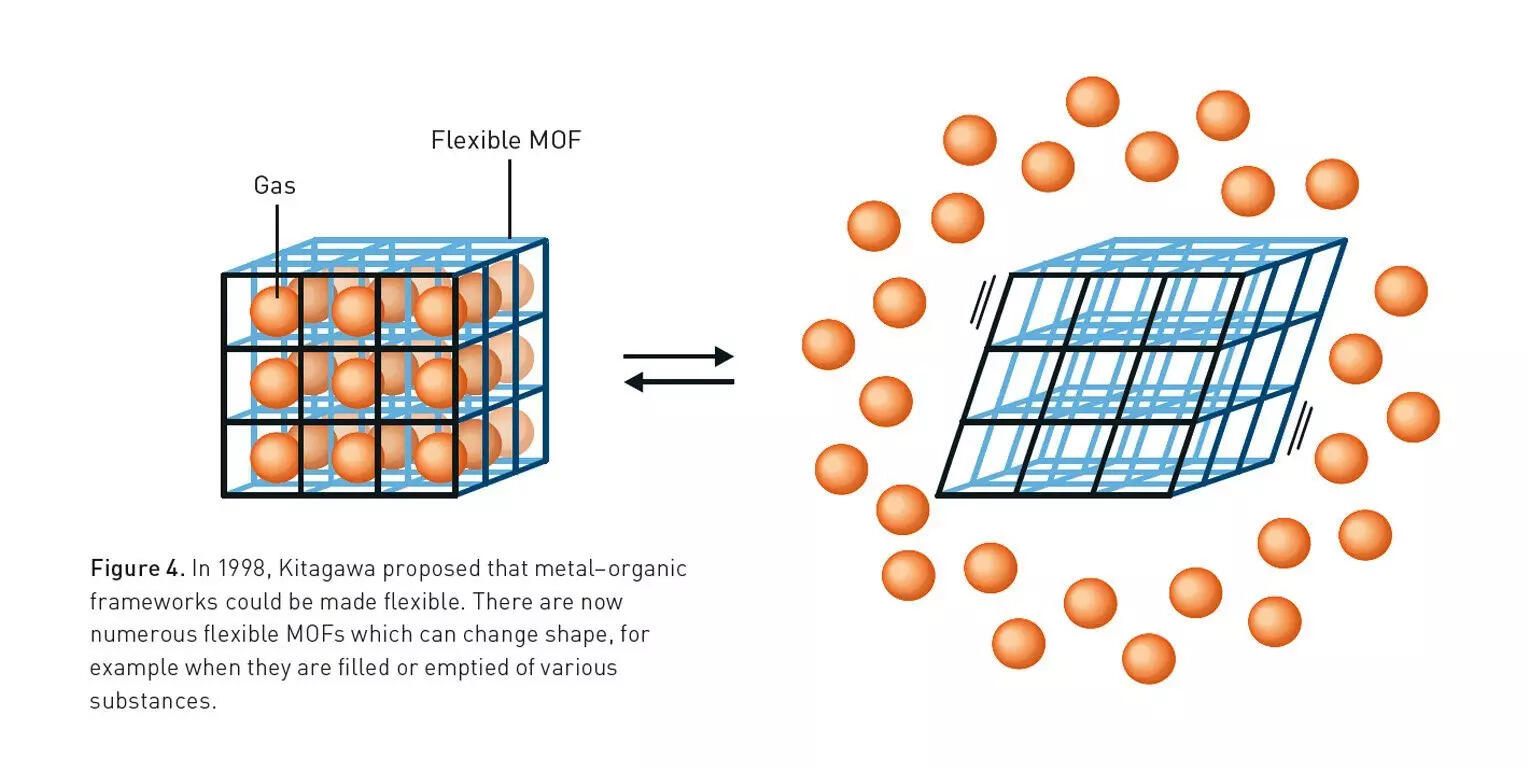
Next came Susumu Kitagawa from Japan — a man whose motto could’ve been printed on a Zen fortune cookie: “See the usefulness of useless things.”In the 1990s, he built strange, floppy materials full of holes that didn’t do anything practical. Funders rolled their eyes. But Kitagawa persisted. In 1997, he created a material that could breathe — it could absorb gases like methane and oxygen and then exhale them without breaking apart.Later, he realised that these frameworks could be soft and flexible, not rigid like rocks. They could expand and contract, almost like living matter. Chemists started calling them “soft porous crystals.”Kitagawa had turned a boring solid into something dynamic — a material that literally inhaled chemistry.
The Dreamer Who Built MOFs Big Enough to Hold a Football Field
And then there was Omar Yaghi, a Jordanian-born chemist who grew up in a one-room house without electricity. When he was ten, he broke into his school library, opened a random book, and fell in love with molecular diagrams. In 1999, Yaghi unveiled MOF-5, a masterpiece — stable, heat-resistant, and so porous that a few grams of it had the internal surface area of a football pitch. Imagine cramming an entire stadium into a spoonful of powder. That’s MOF-5. This structure could trap gases, store hydrogen, and survive 300°C without collapsing. It was chemistry’s version of a Tesla — sleek, futuristic, and showing up traditional materials like zeolites for the dinosaurs they were.
What These Materials Can Actually Do
Once Yaghi and Kitagawa proved MOFs could be built reliably, the field exploded. There are now tens of thousands of them — each designed for a specific job.
- MOF-303 pulls water out of thin desert air — literally condensing drinking water overnight.
- MIL-101 stores hydrogen or carbon dioxide and breaks down pollutants in water.
- UiO-67 filters out “forever chemicals” like PFAS.
- ZIF-8 can mine rare metals from wastewater.
- CALF-20 captures carbon dioxide from factory exhausts.
In short, these things can save the planet — or at least delay its funeral.
Why It’s a Big Deal
Chemistry, for most people, is about explosions and coloured liquids. MOFs are quieter but far more revolutionary. They’re programmable matter — materials that can be customised atom by atom for whatever we need. They can trap CO₂, clean toxic water, store renewable fuels, and even deliver drugs inside the body. And the best part? They can be mass-produced cheaply and recycled endlessly. If plastic was the defining material of the 20th century, MOFs might be the material of the 21st.
From Breaking Bad to Breaking Good
Think of it this way: Walter White made crystal meth. These guys made crystal life. Robson built the foundation. Kitagawa gave it breath. Yaghi made it immortal. Together, they didn’t just mix chemicals — they redesigned space itself. So if you ever see a chemist smiling into a powder and muttering “beautiful,” don’t worry — it’s not meth. It’s a MOF. And it just might save your lungs, your planet, and your glass of water.So to paraphrase Heisenberg: Say their names – Richard Robson, Susumu Kitagawa, and Omar Yaghi.

